Abstract
This proposal describes novel, tested and patented products – Nanophase Manganese Oxide (NMO-V) and nanophase Manganese Oxide coating to clay (NMOC-V) with violet colored (labeled with V suffice) – that destroy biological and chemical warfare agents (BCWAs). The physical, mineralogy and chemical properties of the product, which is strong Lewis acids in that they attack lone pairs of electrons from organic compounds containing P, S, N and O groups and good Friedel-Craft acylation reactions, will be discussed. The advantages of NMO/NMOC-V and the chemical reactions will be examined in aqueous and non-aqueous solvents. Specifically, the reactions with surrogate chemical warfare agents (CWAs) such as sulfur mustard and sarin, and biocides will be addressed. This will enable degradation of the chemical warfare agents (CWAs) stockpiles without hazardous toxic incineration. In addition to their direct application CWAs to destroy the chemical activity of the CWAs, the products can be applied to fabrics such as clothing, wipes and tarps and offer the same CWA inactivating and protective properties. Data on biocidal activity against unicellular organisms (including algae and bacteria) will be presented. The uses of the NMO products in environmental remediation, the military and civilian applications will be discussed. Advantages compared to other approaches will also be addressed including product safety as NMO-V and NMOC-V are non-hazardous and non-toxic to humans and animals.
- Background
This section provides a review about the mineralogy and chemistry of nanophase Mn oxide (NMO) and nanophase Mn oxide coated clay (NMOC). As the product is violet colored, they will be referred to as NMO-V (for nanophase Mn oxide-violet colored) and NMOC-V (for nanophase Mn oxide coated clay- violet colored). As discussed later in the section, the violet color provides quality assurances that the product is stable, as it changes to a brown hue after it has chemical reaction occurs (chemically destroys or degrades).
1.a. Nanophase Mn Oxide (NMO) Synthesis and Properties
The stabilization of nanophase Mn oxides violet colored (NMO-V) states was initiated a couple of years ago by our laboratory. Since then, we have demonstrated that NMO-V is a strong Lewis acid (electrophilic compound) in that it attacks lone pairs of electrons from organic compounds containing P, S, N and O groups, and good Friedel-Craft acylation reactions. Further, NMO-V is effective in polar and non-polar solvents as the electrons exchange reaction occurs on their surfaces. A US patent (No. 6,953,763) by Vempati and Son (2006) was granted for the process of manufacturing nanophase Mn oxide (NMO) using phenylenediamine (PDA).
1.b. Mn-Oxidation States and Color
In nature, Mn mostly exists as Mn(II) and Mn(IV), whereas Mn(III) and Mn(VII) tend to disproportionate into the above mentioned stable oxidation states. In minerals, a Mn(II) octahedral coordinate state is identified by its visible and near-infrared absorption spectrum, consisting of a sharp band near 412 nm and two weak bands at longer wavelength (Rossman, 1988). There are several reports concerning the stabilization of various Mn oxidation states in solution but none on stabilization on the solid phase. In the chemical literature there are references of the predominant mineral colors due to Mn oxidation states. For example, octahedral Mn(II) in a mineral is pink but in tetrahedral sites it is a yellow-green color. When Mn(III) is present in octahedral sites is either red/lavender or green/turquoise. Mn(IV)- and Mn(VII)-minerals are brown to black and violet, respectively.
This work is based on our observations recorded during a study of clay and phenylenediamine (PDA) interactions. The studied clay was an expansive type (montmorillonite) mined from Gonzales, TX that contained Mn(II) either in the structure and/or as Mn-oxide impurities.
The various supported Mn oxidation states were obtained as follows:
a). Mn(II) Oxide (Pink): This was synthesized by adding 1,2-phenylenediamine dihydrochloride to the Mn oxide and/or Gonzalez clays. Color of the mineral is beige to pink.
b). Nanophase Mn Oxide Coated Clay Green Colored (NMOC-G): This was synthesized by adding 1,4-phenylenediamine dihydrochloride to Mn oxide coated clays and/or Mn-bearing clays. It is used in reduction and oxidation reactions.
c). Nanophase Mn(IV) Oxide Coated Clay: No PDA isomer stabilized this oxidation state. Based on scientific literature the color of the mineral: brown to black depending on crystalline and amount of Mn(IV) present.
d). Nanophase Mn Oxide Coated Clay (NMOC-Violet): This is synthesized by adding 1,4-phenylenediamine to Mn oxide and/or Gonzalez clays (NMOC-violet). Effective against destroying lone pairs of electrons present in S and N compounds, e.g., mercaptan and cyclohexylamine. Color of the mineral is violet.
e). Nanophase Mn Oxide Coated Clay (NMOC-Turquoise): This is synthesized by adding 1,4-phenylenediamine to Mn oxide coated solid support media and setting the pH in the range of 5.0 to 6.5. The color of the minerals is turquoise. We have shown proof-in-principles that this mixed valence Mn mineral destroys quaternary compounds (quats), e.g., tetramethyl ammonium chloride and tetrapropyl ammonium hydroxide.
We plan to confirm this using a cyclic voltammeter that would enable us to improve on the visual observations and Mn oxidation states. The change in color of NMO and/or NMOC will helps us to use as a chemical sensor for exhaustive of reactions (See Section 2.h.).
1.c. Chemistry of Phenylenediamine (PDA)
Phenylenediamine (PDA) has a structure shown in Figure 1. The o- (1,2-), m- (1,3-), and p- (1,4-) isomers are inexpensive and readily available commercially. The solubilities of these compounds in water are 3.0, 25, and 3.8 g per 100 mL, respectively.
1.d. Solid-Support Materials
The montmorillonite, L 10 Bentonite clay procured from Southern Clays, Gonzales, TX (NMOC) or even natural and synthetic zeolites (NMOZ) can be coated for this purpose is used as a solid support material.
1.e. Degradation of p-Phenylenediamine (PDA) Compounds
p–Phenylenediamine compounds are used in hair dyes, textile dyes, Kevlar manufacturing – used in bullet-proof vests, and fire-proof materials. Based on our extensive literature search these compounds are classified as allergens; therefore, this study was conducted to determine the presence of any residual amount, which may be critical for safe handling.
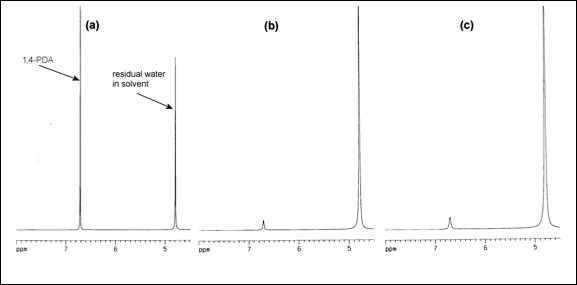
Fig. 2. (a) Before reaction, (b) and (c) loss of 1,4-PDA after synthesis of zeolite (ZSM-5)-supported Nanophase Manganese Oxide on ZSM-5 (NMOZ (violet)).
The consumption of PDA during the preparation of zeolite (ZSM-5) supported nanophase Mn oxide violet colored (NMOZ-V) was observed using 400 MHz 1H NMR spectroscopy techniques. A known concentration of 1,4-PDA was prepared in D2O and the 1H NMR spectrum of this solution was measured (Fig. 2a). The identical concentration of 1,4-PDA/D2O solution was prepared and utilized to synthesize ZSM-5-supported colored violet (NMOZ-V) in two separate experiments. The reaction mixtures were filtered through Celite and glass wool, and 1H NMR spectra were obtained of the filtrates. In both cases, the concentration of PDA was clearly diminished compared to the standard, confirming that PDA was being consumed in the reaction (Fig. 2b and 2c). Synthesis of clay-supported NMOC-V also resulted in considerably diminished levels of 1,4-PDA. No other peaks were present in the 1H NMR spectra, suggesting that either the PDA is bound to the solid support in some fashion or is being converted to an organic product which is insoluble in the highly polar D2O. The degraded products appear to be organic in nature but the exact identity of the by-products will be ascertained as part of the proposed research. This suggests that the final compounds can be handled safely. The FTIR of the dried NMOC-V aqueous slurry did not show the presence of any amine and/or ammonium bands. The PI has handled the final product for silverware cleaning (removes tarnish) without gloves with evidence of no rash or skin allergy.
1.f. Characterization
We have selectively characterized the material using X-ray diffraction, Fourier transform infrared spectroscopy (FTIR), thermo-gravimetric analysis (TGA) and scanning electron microscopy. The NMO violet (with no support) x-ray diffraction shows of amorphous mineral plus a minor Mn mineral. Further, the FTIR spectrum did not indicate any amine based compound or any bands resulting from NMO-V formation using Mn(II) compound. NMO (Green) at pH 3, without support, cannot characterize because Mn dissolved at that pH; therefore, the research was done with NMO-V.
The preparation and identification of oxidation states of supported Mn have been presented. One attractive attribute for the use of the final products in practical applications is the ease with which they can be made through the procedures described. Another important attribute is the stability of the products. Within the solid supports, clays and zeolites, the oxidation states of Mn maintain their stability until transferred to the reaction site. Supported Mn can be delivered to essentially any environment.
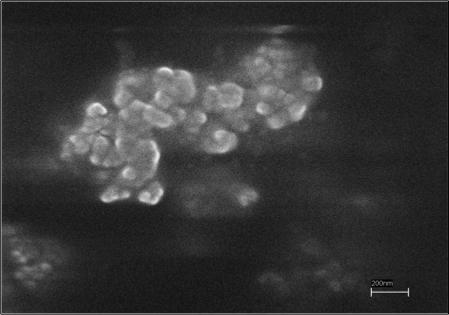
Fig. 3. Scanning electron micrograph of NMO. The scale bar is 200 nm.
The SEM images (Fig. 3) of NMOC-V showed particle size ranging from 50 nm to 100 nm. Since NMOC-G is on clay support no SEM imaging work was performed.
1.g. Optical Spectroscopy
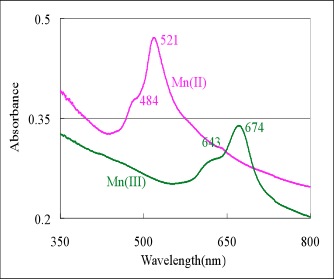
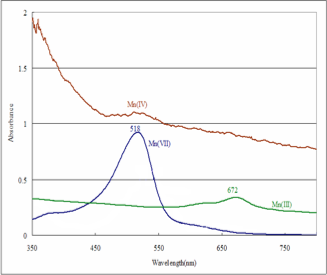
The colloidal Mn oxides coated with clay was stirred in curette and put in optical spectroscopy was recorded (see Fig. 3). The optical spectroscopy in the visible region (300 nm to 700 nm) indicated that the various Mn oxidation states have distinct features (see Table 1). The colloidal Mn(II) oxide has bands at 521 nm (dominant) and 474 nm (shoulder), colloidal NMOC-G at 674 nm (sharp) and 643 (shoulder), colloidal Mn(IV) oxide has no band and colloidal NMOC-V at 518 nm (broad) (Fig. 4 and Table 1). Figure 4 shows that the NMOC-V at 518 nm (blue line) disappears upon its reduction to colloidal Mn(IV) oxide (brown line in Fig 4.-Right).
1.h. Smart Sensor
The Mn oxidation position, based on optical spectroscopy was not unique because the color on tetrahedral and octahedral position. The Mn oxidation will be confirmed by cyclic voltammeter in future. Therefore, we used distinct color of NMO. This property can be exploited to make an optical sensor to monitor the reaction and/or exhaustion of the starting material (Table 1).
Left
Right
Fig. 4. Optical Spectroscopy: i) Left Graph of Spectra of Mn(II) (pink) and NMOC-G, and ii) Right Graph of Spectra Mn(IV) (brown) top, NMOC-G center and NMOC-V in bottom in the visible region.
| Table 1. Mn Wavelengths of Different Colors. | |||
| Color | Nomenclature | Wavelength (nm) | |
| Pink | NMO (pink) | 521D | 474Sh |
| Green | NMO-G (Green) | 674B | 643Sh |
| Brown/Black | NMO (Brown/black) | – | – |
| Violet | NMO-V (violet) | 518B | |
| D=Dominant; Sh=Shoulder; B=Broad | |||
1.i. Nanophase Mn Oxide Clay (NMOC) Technology
The novel NMOC was synthesized using p-PDA by Vempati and Son (2006). The advantages are:
- Environmentally friendly.
- Rapid process, and low production costs.
- Site-specific manufacturing or safe transportation in powder or slurry form.
- Non-corrosive and compatibility with several coated materials.
- NMOC has the special ability to destroy CWA in an effective and safe manner.
o Broadcast or spray the contaminated area resulting in complete destruction of CWA.
o Easy to handle with no specialized equipment and with minimum supervision.
o The NMOC-V to brown/black color indicating its consumption during the reaction, which enables workers to add more of it during decontamination process. Also, a sensor and/or smart fabrics can be developed to determine the exhaustion of the starting material.
o No special containers or protocol needed for transportation.
o Product manufactured in powder, pellet, slurry or aerosol forms.
o In NMOC-V form the material is highly dispersed which results in increased contact between the chemical warfare agents (CWA) and surfaces; thus, improving the degradation efficiency.
- NMOC-V dispersed and remained suspended in solution until they are reduced to NMOC (brown/black).
- Long shelf life: the material is stable even after three years or more of synthesis, and
- Operates in polar and non-polar solvents and has good permeability.
1.j. Solid Support Materials and Advantages
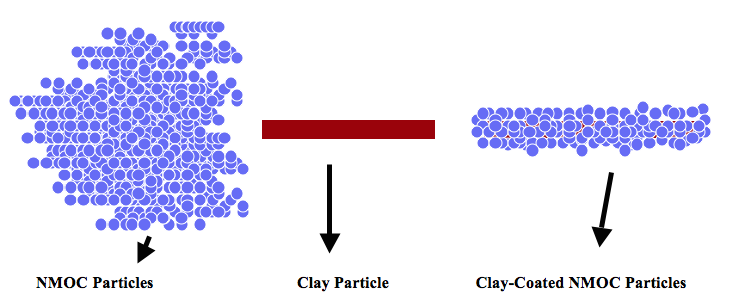
Fig. 5. Nanophase Mn Oxide (NMO) coating on clay surfaces.
The following support materials were used to prepare the various solid-supported Mn oxides (Figure 5).
i) L 10 Bentonite Clay is a montmorillonitic hydrophilic clay procured from Southern Clays, Gonzales, TX, and
ii) Coating NMOC will not cause health problem with small particulates lodging in lungs with contaminated air.
1.k. Reaction Chemistry with NMOC (Violet or Turquoise Colored)
This important section describes the benefits of the chemistry of NMOC-V.
To date these applications have been tested to differentiate NMOC-V and KMnO4 reaction mechanisms with selected compounds containing lone pairs of electrons:
- Malodor removal from mercaptan by NMOC-V. The S odor removal applications are for treating drinking water and domestic and industrial wastewaters. In the case of NMOC-V, the oxidation reactions of the organics occur at the surface; hence, the polarity of the solvent is not an issue (Hua et al., 2006).
- NMOC-V degrades (without by-products) cyclohexylamine (CHA). (The reaction with KMnO4 results in formation of cyclohexanone).
- NMOC-V degrades (without by-products) cyclohexanone present in chemical dye industry waste streams. (No reaction with KMnO4).
- NMOC-V destroys 2-chloroethyl ethyl sulfide (sulfur mustard gas analog) and dimethyl methyl phosphonate (sarin gas analog), both are simulated chemical warfare agents (CWAs); therefore, NMOC-V has the ability to treat chemical weapon stockpiles and make an effective filter for protective gas masks (Vempati et al, 2006; Vempati et al., 2011 [U.S. Patent 8,084,662]).
- NMOC-V reacts vigorously with hydrogen peroxide (H2O2) to form O2. This is one of the characteristics that can be used in H2O2 based explosive. The color change from violet to brown indicates that the original material has been spent.
- NMOC-V converts hypochlorite to Cl2 gas. Hypochlorite is used for household cleaning, bleaching and swimming pool cleaning.
- Converts thiol group to disulfides (Gondi et al., 2010). NMOC-V is a catalyst, which can be reused more than four times. The applications of disulfides are: perfume industry, odorless garlic tablet and pharmaceutical industries (Gondi et al., 2010).
- A number of Friedel-Craft reactions were worked in our laboratory using NMOC-V as a catalyst. This catalyst can be recycled several times, better than classical AlCl3 catalyst, improves energy efficiency and make it was a green technology (Vempati and Gondi, unpublished).
- Destroys quaternary ammonium compounds present in the wastewaters of electronic and semiconductor industries using Mixed NMOC (Turquoise).
Therefore following conclusion may be drawn:
The organic compounds containing lone pairs of electrons react on the surfaces of the NMOC-V probably at the Lewis acid sites. This is further supported by the change of the NMOC-V to brown/black Mn(IV) oxide. KMnO4, a salt, is effective only in polar solvents, and/or when it is deposited on a cation exchanger; such as, clays, zeolites, etc
2. Chemical Warfare Agents (CWA) Reactions with NMOC-V Compared to KMnO4
This research was initiated to determine the effectiveness of NMOC-V in removing CWA surrogates, again the chemistry of the these products and/or byproducts are such that they contain, N, S, P and O lone pairs of electrons which makes them suitable candidates (Vempati et al., 2011 [U.S. Patent 8,084,662]).
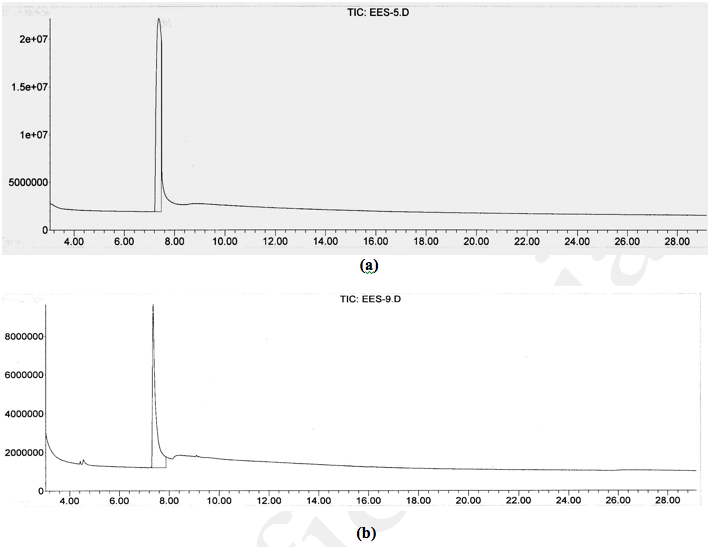
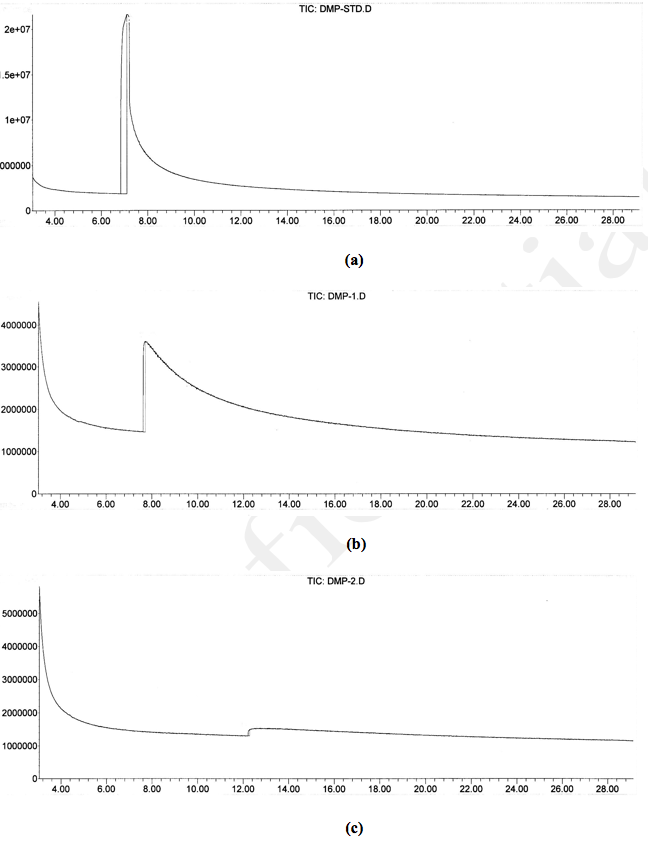
The testing was conducted using 2-chloroethyl ethyl sulfide (CEES), sulfur mustard analog, and dimethyl methyl phosphonate (DMMP), sarin compound analog. The use of KMnO4 is again to illustrate the differences in reaction mechanisms. A 5,000 ppm of CEES solution dissolved in dichloromethane was prepared for the experiment. Two gms of KMnO4 and one gm NMOC-V (heated to 110ºC) were added to separate conical flasks, each containing 20 mL of 5,000 ppm CEES. The reaction destroyed 120 mg of CEES per kg of NMOC-V but no reaction with KMnO4. Flasks were placed in a bath maintained at 70ºC and the contents were refluxed for 3 hrs. Balloons attached to needles were placed at the top of the capped reflux units to collect evolved gases. Considerable amount of gas evolved in the NMOC-V flask within 15 min of reaction but no gas was generated in the KMnO4 flask. The Gas Chromatography and Mass Spectrometer (GC-MS) spectra of original starting material, KMnO4 and NMOC-V reacted samples were collected. The original starting material and KMnO4 treated sample showed a peak at 7.36 min with the corresponding Mass spectrum at mass 124, indicated the presence of starting material (Fig. 6a,b). The CEES reacted with NMOC-V did not contain any GC peak indicating its complete destruction (Fig. 6c). The trapped gas extinguished a candle light indicating the likely presence of CO2 and/or CO. Similarly, when DMMP was reacted with NMOC-V and KMnO4, the former destroyed the compound as evidenced by the absence of 7.09 min peak with a mass of 124 (see Fig. 7a,b,c). The reaction destroyed 22 mg of DMMP per kg of KMnO4 and 250 mg of DMMP per kg of NMOC-V.
2.a. Pure Gonzalez Clays
The pure Gonzalez clays provided by the vendor did not destroy either the CEES or DMMP compound under similar concentrations and conditions.
Fig. 6. a) Starting material containing 5,000 ppm of 2-chloroethyl ethyl sulfide (2-CEES), b) Two gm of KMnO4 reacted with 5,000 ppm of 2-CEES, and c) One gm of clay coated with 10% NMOC-V reacted with 5,000 ppm of 2-CEES. The absence of 7.36 min peak indicates the destruction of the CEES compound. Pure clay did not destroy CEES.
Fig. 7. a) Starting material containing 5,000 ppm of dimethyl methyl phosphonate (DMMP), b) Two gm of KMnO4 reacted with 5,000 ppm of DMMP, and c) one gm of clay coated with 10% NMOC-V reacted with 5,000 ppm of DMMP. The absence of 7.09 min peak indicates the destruction of the DMMP compound. Pure clay did not destroy DMMP.
3. Microbial Work
Fig.8. Controlalgae(Chlamydomonasreinhardtii–1×106 cell/ml) and NMOC-Vtreatedalgae (right). Note that the clarity of the solution and accumulation of killed algae at the bottom of the tube. Upon reaction with the algae, both product and algae drop to the bottom of the tube. In addition, the product changes from violet to brown. The color change can serve as a valuable indicator of activity as untreated NMOC-V remains suspended and green-colored.
Preliminary experiments have indicated that the product is active within a range of 3-10ppm; this corresponds to an active ingredient of0.3–1ppm. Experiments with different concentrations of NMOC-V and algae suggest that a linear relationship exists between the amount of NMO-V added and the amount of algae killed. When levels of algae were higher than a certain threshold relative to a fixed amount of NMO-V, regrowth occurred. This maybe due to the requirement of direct contact between the product and the algae.
Comparisons were made with copper sulfate added to separate tubes containing the same concentration of algae (1×106 cells/ml) as the experiments conducted with NMOC-V (Fig.9). As was similar to the NMOC-V, the algae clumped and sunk to the the bottom of the tube; however, in contrast to the clearing effect of NMOC-V, the solution turned blue, due to the cupricion. Microscopic evaluation of the sediment revealed that the cells treated with NMOC-V appeared as hollow shells suggesting that the plasma membrane had been destroyed leaving only the cell wall, which has a high protein content. In contrast the cells treated with copper sulfate appeared intact with full cellular contents. From this observation, it appears that the mechanisms of the two algaecides are different.
Fig.9. Comparison of reaction with algal cells treated with NMOC-V (second tube from left) and copper sulfate (third tube from left).
In addition to its algaecidal activity, NMOC-V is effective against the bacteria Escherichiacoli, too(Fig. 10). This suggests that the activity is broad spectrum, possibly due to its oxidizing potential.
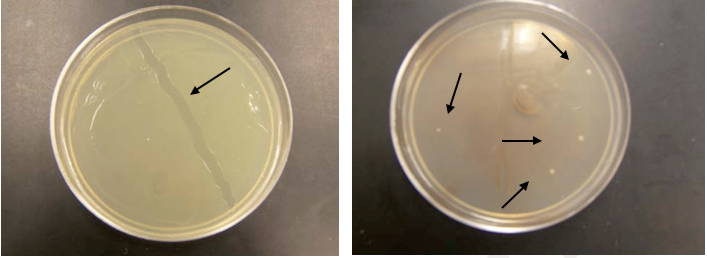
Fig.10. Bacterial growth on solid media from cells not treated (left) and treated (right) with NMOC-V.
Several test tubes containing LB media were inoculated with a single colony of E.coli and allowed to grow for 14 hrs at 37 degrees C. Bacterial suspensions were treated or not treated with NMOC-V. Tubes were returned to the incubator for another 14 hrs. One tube was kept untreated as a control. The tubes are then plated on LB agar with 100µl of sample from the tubes. The Petri plates were returned to the incubator overnight. The pictures shows that the control sample grew as expected and produced lawn the LB media plate (Photo on Fig. 10 (left)-note the strip of bacteria removed (arrow) using a transfer loop). The tubes that were treated with the NMOC-V showed significant inhibition of growth. The plate yielded only four colonies. Assuming conservatively that the cells were in late log phase and there would be approximately 1×108 cells, the if all cells survived–as in the control-one would expect 1×106 cells to be delivered to the plate (100 µl sample), resulting in the observed lawn. Since only 4 cells (Right, see arrows) grew the survival rate was 4/(1×106) or 0.0004%.
Our most consistent results have been with NMOC-V treated fabrics in which the growth of cells delivered to the treated fabrics has been repressed. Treatment of fabrics coated with NMOC-V with a suspension of algae demonstrated that the fabrics had excellent algaecide activity (see Figure11). Cotton fabrics treated with NMOC-V were cut into approximately 0.5 in x 0.5 in squares. The samples were inoculated with 200µl of a three day culture of Chlamydomonasreinhardtii (green algae). The samples were kept in separate Petri dishes and sealed with parafilm. The dishes were kept at 20-25 degrees C for 24 hrs. The individual fabric samples were then placed into 125 ml Erlen meyer flask containing 50 mls of TAP media. The flasks were placed on a platform shaker for three days in full light and at 20-25 degrees C with the shaker set at 130 rpm. As seen in the photo the two flasks on the left containing the cotton not treated with NMOC-V supported vigorous algal growth.The two flasks on the right containing the NMOC-V treated cotton showed no sign of algal growth indicating algaecidal activity. (Fabric clothes treated with NMOC-V courtesy of Dr. G. S. Bhat).
We continued these experiments to determine if NMOC-V treated fabrics suppressed bacterial and algal growth. Three different non woven fabric samples were used in this study to coat the NMOC-V (Vempati and Bhat, 2012 [U.S. Patent 8,163,036]. They were 80 gsm needle-punched cotton non woven, a through air bonded cotton/PLA web and a melt blown polyurethane web. Needled cotton web was supplied by the Cotton Incorporated, Raleigh, NC, and the melt blown PU and cotton/PLA thermal bonded webs were produced at the University of Tennessee, Knoxville,TN (UTK). These webs were either produced to, or cut to the width to process continuously in the Mathis padding drying equipment.
Fig.11. Results from non treated and treated fabrics inoculated with algae.
All the padding experiments were carried out using the Mathis 2- roll laboratory padder type VFM, 350 mm wide. The fabric was fed through a feed roller to a trough containing the Mn-suspension, then pressed using the pressure rollers to remove excess of the suspension and get a good coating. The coated samples were dried/cured at150°C using the Mathis KTF dryer.
4. Research and Developmental Work
The following R&D work will be demonstrated for the efficacy and utility of the NMO-V and NMOC-V products:
- Demonstrate destruction of CWAs stockpiles, i.e., sulfur mustard, Sarin gas and other CWAs’ with Lewis bases.
- Manufacture and test protective garments for soldiers and civilian populations (U.S. Patent 8,163,036). Incorporate NMOC-V in soldiers’ clothing for protection against CWAs so as to provide greater flexibility/movement for the army to operate during terrorist attack. Other potential applications are to incorporate into hospital beds and blankets to destroy odors and pathogens.
- Make under garments for the combat soldiers to destroy odor and pathogens (U.S. Patent 8,163,036).
- Destroy CWAs and Toxic Industrial Chemicals (TICs) present in air, water, and soils. The current activated C based fabric only sorb these chemicals; therefore, the contaminated clothing has to be incinerated before disposal.
- Degrade ammunition wastes containing lone pairs of electrons (pink water).
- Develop effective skin lotion decontamination against CWAs and TICs.
- Manufacture decontamination spray and wipes for contaminated interior spaces, vehicles, aircrafts, sensitive equipment, etc.
- Develop mat/wipe technology for treating TICs spills, which can be utilized by Haz-Mat and Spill response team.
- Work on a water filtration unit for drinking water supplies contaminated with CWAs, TICs and pathogens.
- Develop technology for the decontamination of existing CWAs stockpiles globally.
- Develop sensors for peroxide based explosives (such as triacetone triperoxide (TATP), a non-N based explosive, and hexamethylene triperoxide diamine (HMTD) with low false alarms.
- Produce wipes to prevent and/or destroy algal and bacterial growth.
References
Gondi, S. R., D. Y. Son, E. Biehl and R. K. Vempati. 2010. Easy and Rapid Method Synthesis for Disulfide by Nanophase Manganese(VII) Oxide Coated with Clay. Phosphorus, Sulfur and Silicon, 185:34-39.
Hua, X., J. E. Kearney, R. K. Vempati, D. Y. Son, G. Reddy and E. R. Biehl. 2006. Innovative Nanophase Mn(VII) Oxide Technology for Deodorizing Sulfur Based Compounds from Waters. Texas American Water Works Association. Austin, TX. April 04-07, 2006 (Conference Proceedings).
Rossman, G. R. 1988. Optical Spectroscopy. In Spectroscopic methods in mineralogy and geology. (ed. Hawthorne, F. C.) Reviews in Mineralogy, 18, 207-254.
Vempati, R. K. and D. Y. Son. 2006. Solid Support Stabilized Mn(III) and Mn(VII) and Method of Preparation. U.S. Patent No. 6,953,763.
Vempati, R. K., G. S. Bhat, and R. W. Wagner. 2007. Nanophase Manganese Oxides Coated Nonwoven Applications. Cotton Utilization Conference: Nonwovens. Beltway Cotton Conference Proceedings, New Orleans, LA.
Vempati, R. K., R. S. Hegde, E. R. Biehl and D. Y. Son. 2011. Degradation of Chemical Warfare Agents using Mn(VII) Oxide With- and Without-Solid Support. U.S. Patent 8,084,662.
Vempati, R. K. and G. S. Bhat. 2012. Nanophase Mn(VII) Oxide (NM7O) and Nanophase Mn(III) Oxide (NM3O) Incorporated Nonwovens. U.S. Patent 8,163,036.
Download: Innocentive2013